Define Osmolarity and Tonicity and Explain the Difference Between Them
Osmolarity is the overall concentration of all solutes solution within the solution. We asked students who completed a course in animal physiology to submit an essay explaining what they found most perplexing about this subject and what in-class activities proved most useful to them.
Difference Between Osmolarity And Tonicity
Three termshyerptonic hypotonic and isotonicare used to describe whether.
. Osmolarity is a general term used for all. A membrane that allows a solvent to pass through it but not a solute. I mean there is only one letter different in the words.
Tonicity is the measure of the osmotic pressure gradient between two solutions. To understand hypertonic isotonic and hypotonic you must understand the process of osmosis. Tonicity refers to the measure of the effective osmotic pressure gradient between two solutions.
The solutions being compared have equal concentration of solutes. Whereas molarity measures the number of moles of solute per unit volume of solution. Measurement of osmotic pressure between two solutions.
Osmotic pressure is the pressure of a solution against a semipermeable membrane to prevent water from flowing inward across the membrane. - Osmolarity just a measure of number of solute particles. With osmosis just remember LOW to HIGH.
The osmolality of a fluid is a measure of the number of particles per kilogram of the liquid that they are dissolved in the solute. Osmolality is defined as the number of osmoles of solute per kilogram of solvent. The most rigorous useful definition is.
Tonicity is the osmotic pressure between two compartments and is related to the difference in the concentration of effective osmoles between them. The osmolarity of a solution is usually expressed as OsmL in the same way that the molarity of a solution is expressed as M. The key parts are effective and capacity to exert.
Osmolality is measuring the number of osmoles in a weight kg of solvent. Intracellular and extracellular fluid are two compartments of fluids defined by the relative position of each fluid compartment to the cell membraneThe main difference between intercellular and extracellular fluid is that intracellular fluid is the liquid found inside the cell whereas extracellular fluid refers to all the. Osmolarity is measure the number of osmoles in a volume L of solvent.
When the solvent is water osmolarity and osmolality may be nearly the same under ordinary conditions since the approximate density of water is 1 gml or 1 kgL. Osmotic pressure and tonicity often are confusing to people. When thinking about osmosis we are always comparing solute concentrations between two solutions and some standard terminology is commonly used to describe these differences.
If a solute cannot pass through a plasma membrane but remains more concentrated on one side of the membrane than on the other it causes osmosis. Osmolarity is greater than body fluid. Tonicity is the effective osmolality and is equal to the sum of the concentrations of the solutes which have the capacity to exert an osmotic force across the membrane.
Both are scientific terms pertaining to pressure. Now lets define what solutions tonicity can be. Define osmolarity and tonicity and explain the difference between them.
Tonicity is a term used for substances that can not cross the cell membrane non permeable. Osmolarity is the measure of osmotic pressure of a solution. Hypertonic isotonic and hypotonic are subcategories of crystalloid.
In animals this process is brought about by osmoreceptors which can detect changes in osmotic pressure. What is the difference between Molarity and Osmolarity. Understanding osmolarity and tonicity is one of the more challenging endeavors undertaken by students of the natural sciences.
The key difference between tonicity and osmolarity is that the tonicity measures only the concentration of non-penetrating solutes through a semipermeable membrane while the osmolarity measures the total concentration of penetrating and non-penetrating solutes. In simpler terms it is roughly the. Main Difference Intracellular vs Extracellular Fluid.
The measurement is given in milliosmoles per kilogram or mOsmolkg for short. The difference is subtle between osmolarity and osmolality. - Tonicity influenced by type of particles in solute -- ability to cross membrane and cause shrinking swelling etc.
The implication is that tonicity is less then osmolality. Tonicity is that the capability of a solution because of which water will interchange into or out of a cell by the method of diffusion is phenomena is named Osmotic Pressure. Osmolarity is less than body fluid.
Tonicity of any solution is associated with its solutions Osmolarity. Tonicity helps understand whether a cell exposed to osmotic pressure will swell shrink or remain of the same size. Osmotic concentration formerly known as osmolarity is the measure of solute concentration defined as the number of osmoles of solute per litre of solution.
Osmolarity is equal to body fluid. The number of particles is measured in milliosmoles which is a measurement widely used in chemistry. Low solute high solvent.
The process of liquid moving across a semi-permeable membrane. A hypotonic15 solution has a lower concentration of nonpermeating solutes than the intracellular fluid ICF. Osmolarity is the measure of solute concentration per unit volume of solvent.
Besides the brain osmoregulators are also. The solution with the higher concentration of solutes. Osmoregulation is a process that regulates the osmotic pressure of fluids and electrolytic balance in organisms.
To conclude it can be said that while osmolarity and osmolality are concentrations of individual solutions tonicity deals with the interaction between two solutions across a semipermeable membrane. The higher the difference in the tonicity between the two solutions the more osmosis transpires. Unit of molarity is mol dm-3 whereas unit of osmolarity is OsmL.
It is expressed in terms of osmolkg or Osmkg. Tonicity is a bit different from osmolarity because it takes into account both relative solute concentrations and the cell membranes permeability to those solutes. Tonicity is the measure of this pressure.
Effective osmoles are those substances which are unable to penetrate the membrane between compartments and therefore they are effective in their contribution to the osmotic pressure gradient. Tonicity is the ability of a solution to affect the fluid volume and pressure in a cell. Humans and most other warm-blooded organisms have osmoreceptors in the hypothalamus.
Molarity means the number of moles of solute particles per unit volume of the solution but osmolarity means the number of osmoles of solute particles per unit volume of the solution.
Difference Between Osmolarity And Tonicity
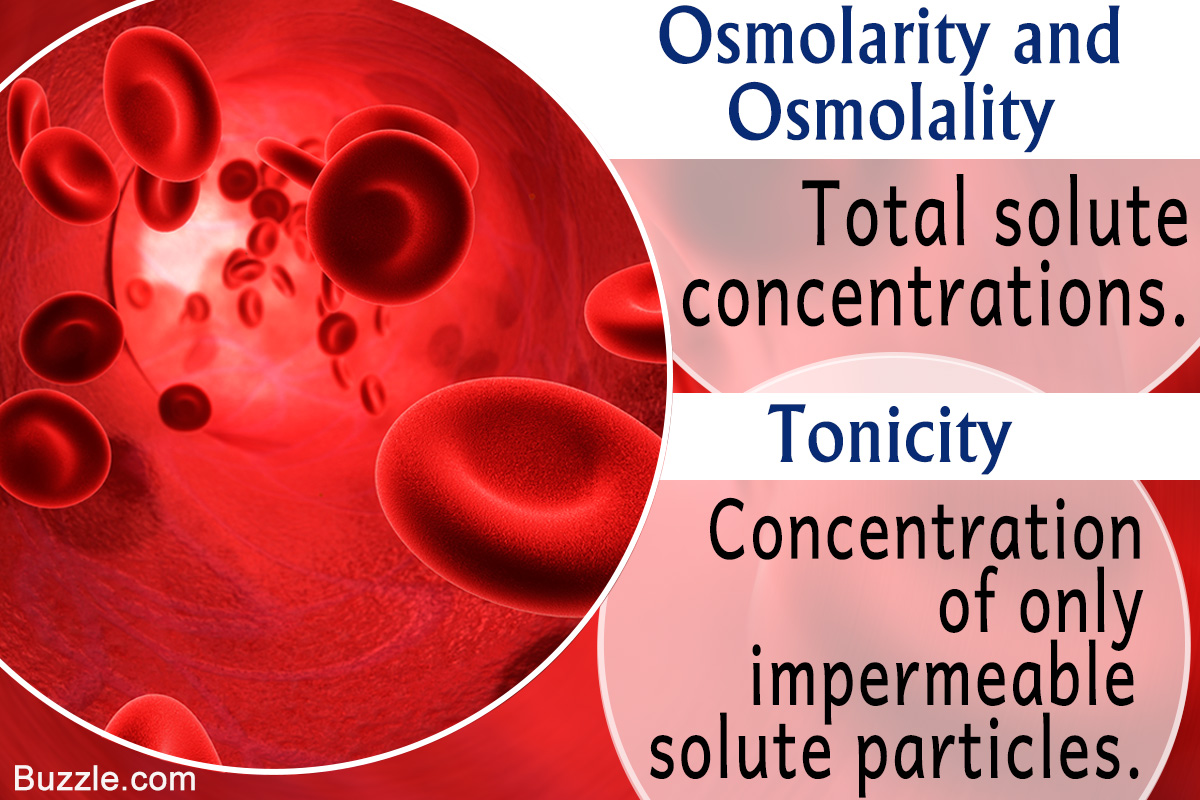
A Comparative Study Of Osmolarity Vs Osmolality Vs Tonicity Science Struck
0 Response to "Define Osmolarity and Tonicity and Explain the Difference Between Them"
Post a Comment